Beyond Pain Management: A Tissue-Specific Approach to Degenerative Spine Conditions
Alex Nichols with Dr. John Saratsiotis
Introduction
Degenerative conditions associated with back pain can benefit from a more nuanced, tissue-specific approach, in contrast with the majority of standard methods. Many of the clients I work with have already exhausted general, non-specific approaches. While these have their place, they often overlook what is required in terms of tissue health, architecture, and capacity to make lasting changes in the spine. While there is a place for general pain management techniques, I want to emphasize the biological structures and processes involved in spine-related degenerative conditions. As FRS® providers, there is much more potential for us to influence these tissues from a training and treatment standpoint.
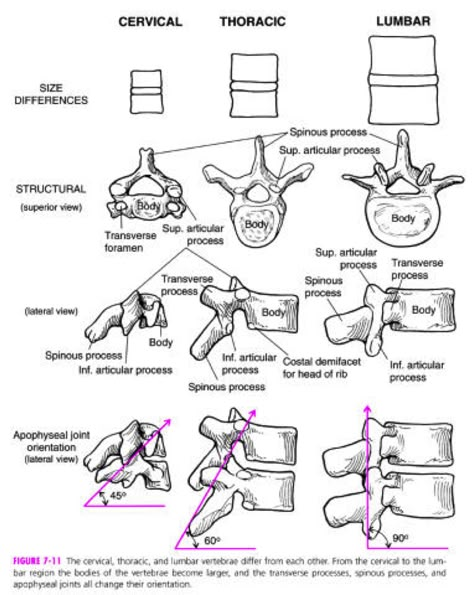
The anatomy of the spine, particularly its connective tissues, can be challenging to conceptualize due to its density and directional variability. To better argue for what we can do for the health of various spine tissues in the context of degenerative conditions, we need to revisit these deeper tissues to fully appreciate where training potential exists.
The Intervertebral Disc (IVD)
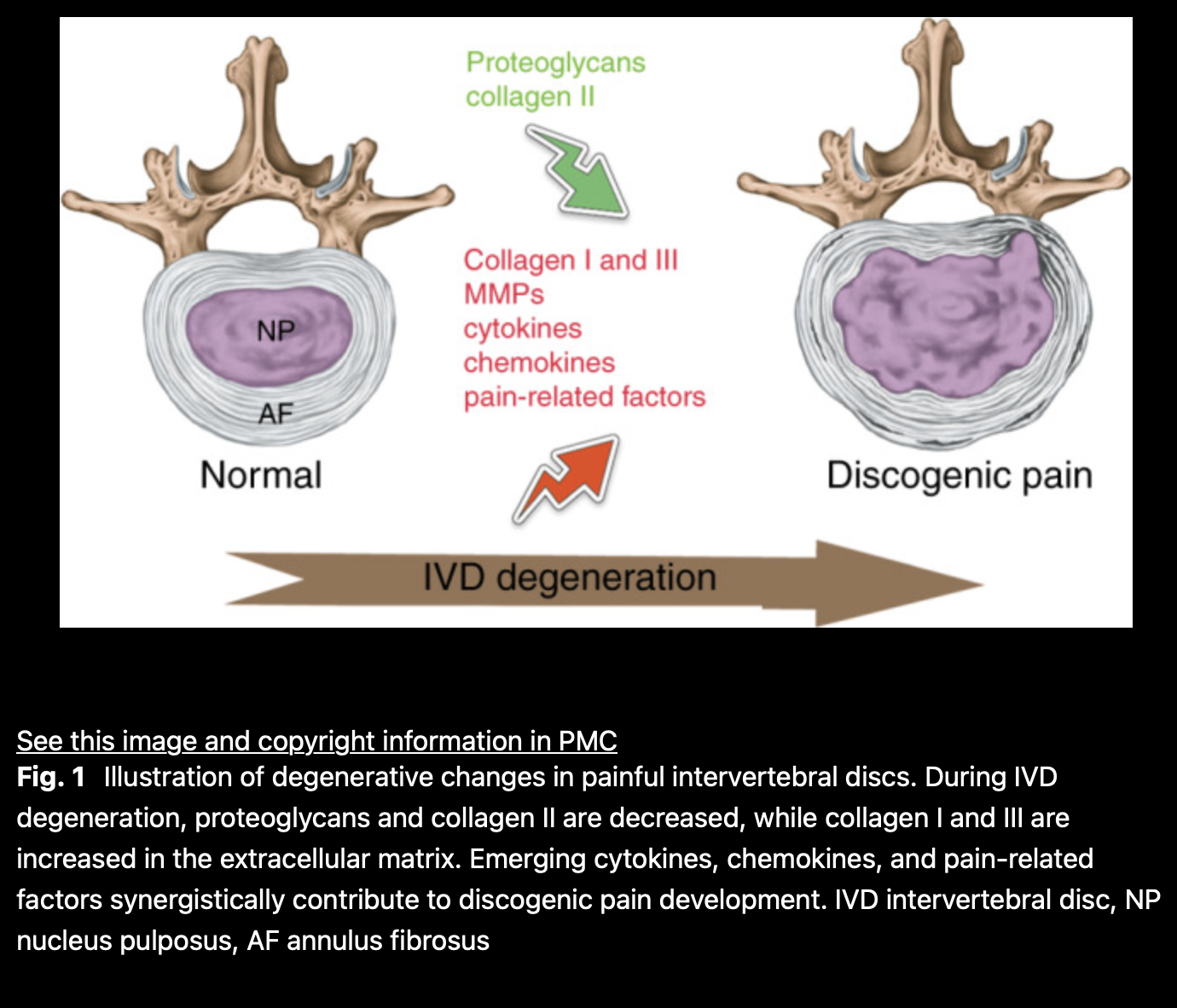
Research has consistently detailed how intervertebral discs (IVDs) break down, a hallmark of spine-related conditions. The intervertebral disc (IVD) is the largest avascular, cartilaginous tissue in the human body. It consists of a colloidal core, the nucleus pulposus (NP), and outer layers of annulus fibrosus (AF), sandwiched between two cartilaginous endplates. The mature NP contains a proteoglycan- and collagen II-rich extracellular matrix (ECM). Under compressive loading, the NP exerts tensile stress on the collagen I-rich AF, supporting the IVD’s capacity to absorb shock and enable spinal motion. (1)
“IVDs undergo progressive degeneration in aging, characterized by inflammation-driven ECM degradation, altered growth factor activity, and the replacement of native disc cells with a heterogeneous cell population. The loss of ECM integrity in the NP results in a loss of water-retaining and load-bearing capacity. These alterations translate into mechanical dysfunction, ultimately leading to disc narrowing, bulging or herniation, causing nerve compression or agitation and back pain.” (1)
It’s important to note that back pain is not the only outcome of degeneration. For example, many individuals with herniated discs do not experience back pain. This is important to register since individuals without back pain dealing with herniated discs could still benefit from a tissue-specific focus for improving the health of trainable spine tissues. The FRS® model accounts for this, offering potential to mitigate degenerative processes by addressing both local and global joint, connective, and muscular tissues in need of training. This is based on the quality and quantity of FRA® findings, along with mico tissue quality findings in FR®. Understanding the role of the ECM, collagen, fibrous elements, and surrounding tissues like the facet joints and endplates is key. While we can’t directly target the cartilaginous tissue in IVDs, I think providers can all agree that improving the health of surrounding tissues would be beneficial. The BioFlow model exemplifies this holistic approach, targeting spine dysfunctions more specifically and proactively. This ecological perspective allows us to develop a comprehensive strategy for tissue health in the spine, addressing the multifactorial nature of degenerative conditions.
The health of ‘the spine’ has a lot to do with the quality of relationships to its parts, as a compromised disc can no doubt affect spine health long-term. Degeneration affects multiple structures across scales of biology, not just the IVD itself. It is these processes over time that can lead to herniation, a common point of discussion with regard to outcome. Not enough attention is given to the multiple biological processes and tissue degeneration that lead to this outcome.
As previously mentioned, “IVD disorders are a major cause of low back pain (LBP), often linked to aging and degeneration. During degeneration, the AF’s integrity is compromised, impairing NP integrity. IVD bulging may occur without rupture of the AF, resulting in contained disc herniation. If the AF and PLL rupture, NP extrusion can occur, leading to uncontained herniation.” (8)
To better understand the anatomy surrounding the IVD, consider the transitional zones between the NP, AF, endplates (EP), and elastic fibers. Recent studies emphasize the role of elastic fibers in maintaining IVD’s mechanical function, providing insights into the multi-scale structure of elastic fibers within the IVD.
The AF consists of lamellae (layers) made of packed collagen fibers connected by elastic fibers. (9)
If you’re grasping the significance of connective tissue in spine degeneration, you’ll recognize the importance of connective tissue-specific interventions as covered in FRS® courses.
Fibrosis in Spine Degeneration
Fibrosis, a pathological event, results from chronic inflammation and excessive ECM accumulation, leading to tissue remodeling. It can alter biomechanical properties and is a significant factor in degenerative spine conditions. “Fibrosis is characterized by increased collagen I and fibronectin deposition, leading to matrix disorganization and scar tissue formation.” (6)
Fibrosis often accompanies chronic inflammatory diseases. In the case of IVD degeneration, fibrosis-like processes involve abnormal fibrotic proteins and fibroblastic NP cells, leading to hardening and scarring of the NP and changes in IVD mechanics. This process has significant implications for spine degeneration.
“In most cases of IVD degeneration, the NP loses ECM, reducing hydrostatic pressure and altering collagen composition. This causes fissures, disc height loss, and spinal instability.” (3) These changes compromise biomechanical function, contributing to back pain.
Fibrotic deposition within connective tissue can be mitigated through training. This represents a shift in treatment strategies, highlighting the importance of connective tissue-specific interventions to address dysfunction more precisely.
Targeted Interventions with FRS®
What exactly can we do differently from standard interventions? We can focus on facet joint interactions, restoring joint space, articular control, tissue quality, and load-bearing capacity—elements that are directly trainable. These interventions may have carry-over effects on elastic and collagen fibers in transitional zones between endplates and annulus fibrosis.
When we appreciate the trainability of connective tissue as taught in the FRS® model, we can better address the need for signaling adaptations at this scale. The long-term impact of these interventions depends on various factors, but the potential for mitigating degenerative spine conditions through targeted training remains significant.
FRS® provides a framework to influence connective tissue quality and improve load-bearing capacity, addressing the multifactorial nature of degenerative conditions. While we must also consider other nuanced conditions like spondylolisthesis, degenerative scoliosis, and stenosis (to name a few), a systems approach allows us to target specific weaknesses in facet joints, connective tissues, and atrophied muscles more precisely than standard approaches.
Degenerative disc issues, bulging discs, and nerve impingements can arise from factors like tears in the annulus or water loss in the nucleus. Over time, this can lead to instability and nerve compression. Intervening at key tissue weak points—whether in facet joints, connective tissues, or muscles—can slow or mitigate degeneration.
As practitioners, we must recognize the importance of connective tissue and understand how to influence it directly and indirectly. This approach advocates for raising the standard of care in spinal health and providing more effective solutions for degeneration and dysfunction.
References
(1) – Sylvie Ricard-Blum, Georges Baffet, Nathalie Théret, Molecular and tissue alterations of collagens in fibrosis, Matrix Biology, Volumes 68–69, 2018, Pages 122-149, ISSN 0945-053X, https://doi.org/10.1016/j.matbio.2018.02.004.
(https://www.sciencedirect.com/science/article/pii/S0945053X17304584)
(3) Chen, C., Zhou, T., Sun, X. et al. Autologous fibroblasts induce fibrosis of the nucleus pulposus to maintain the stability of degenerative intervertebral discs. Bone Res 8, 7 (2020). https://doi.org/10.1038/s41413-019-0082-7
(4) Lyu FJ, Cui H, Pan H, Mc Cheung K, Cao X, Iatridis JC, Zheng Z. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021 Jan 29;9(1):7. doi: 10.1038/s41413-020-00125-x. PMID: 33514693; PMCID: PMC7846842.
(5)Peng, Baogan MD, PhD; Chen, Jindong MD, PhD; Kuang, Zhengda MD; Li, Duanming MD; Pang, Xiaodong MD, PhD; Zhang, Xinyu MD, PhD. Expression and Role of Connective Tissue Growth Factor in Painful Disc Fibrosis and Degeneration. Spine 34(5):p E178-E182, March 1, 2009. | DOI: 10.1097/BRS.0b013e3181908ab3
(6) Castro, A.L., Ribeiro-Machado, C., Oliveira, C.M. et al. Fibrotic alterations in human annulus fibrosus correlate with progression of intervertebral disc herniation. Arthritis Res Ther 24, 25 (2022). https://doi.org/10.1186/s13075-021-02690-w
(7) Steven Tessier, Victoria A. Tran, Olivia K. Ottone, Emanuel J. Novais, Alexandra Doolittle, Michael J. DiMuzio, Irving M. Shapiro, Makarand V. Risbud,
TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression, Matrix Biology, Volume 87, 2020, Pages 94-111, ISSN 0945-053X. https://doi.org/10.1016/j.matbio.2019.10.007.
(https://www.sciencedirect.com/science/article/pii/S0945053X19303877)
(8) Sun Y, Lyu M, Lu Q, Cheung K, Leung V. Current Perspectives on Nucleus Pulposus Fibrosis in Disc Degeneration and Repair. Int J Mol Sci. 2022 Jun 14;23(12):6612. doi: 10.3390/ijms23126612. PMID: 35743056; PMCID: PMC9223673.
(9)Cyril D, Giugni A, Bangar SS, Mirzaeipoueinak M, Shrivastav D, Sharabi M, Tipper JL, Tavakoli J. Elastic Fibers in the Intervertebral Disc: From Form to Function and toward Regeneration. Int J Mol Sci. 2022 Aug 11;23(16):8931. doi: 10.3390/ijms23168931. PMID: 36012198; PMCID: PMC9408956.